You are about to leave EKTERLYHCP.com
You will be redirected to a third-party website
not affiliated with KalVista Pharmaceuticals.
This website is intended for US healthcare professionals.
Attacks in all locations were eligible for treatment2
*Prophylaxis treatments included berotralstat (38%), lanadelumab (33%), and C1 inhibitor replacement (25%).2
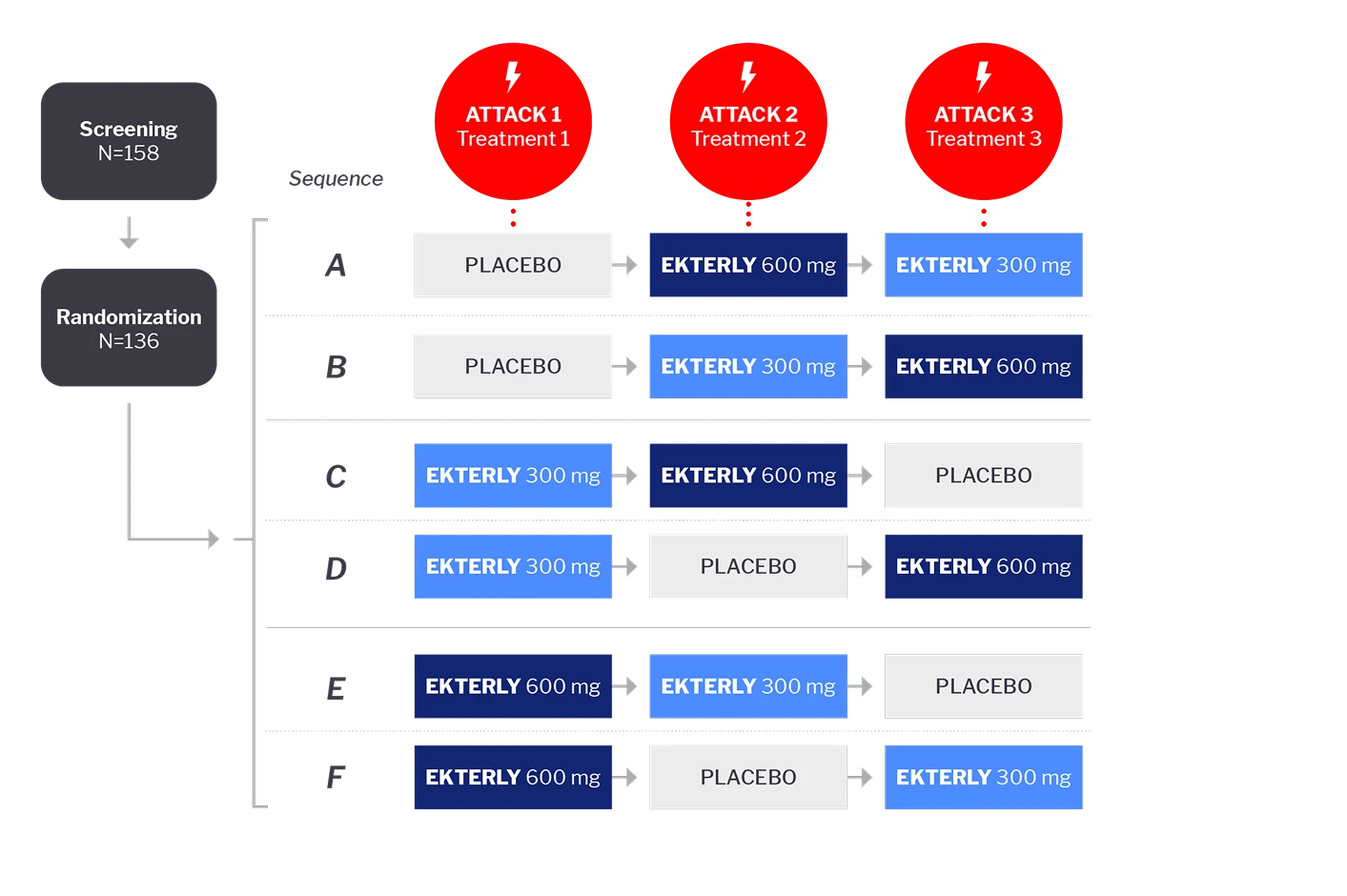
KONFIDENT trial
KONFIDENT was a multinational, randomized, double-blind, placebo-controlled, phase 3, crossover study of 136 patients from 17 countries. Study participants were randomized to receive either placebo, EKTERLY 300 mg, or EKTERLY 600 mg to treat 3 attacks in a 3-way crossover design using 1 of 6 treatment sequences.2,4

PRIMARY ENDPOINT
Defined as a rating of at least “a little better” for at least 2 consecutive timepoints on the Patient Global Impression of Change (PGI-C) scale within 12 hours of first dose
PGI-C (7-Point Scale)4



Patients who reported at least “a little better” had clinically meaningful improvement in attack symptoms6
Median Time to Treatment From Attack Onset2,8
(93 attacks)
41
minutes
(1706 attacks)
10
minutes
 All attacks were eligible for treatment2,†
44.1%
All attacks were eligible for treatment2,†
44.1%
 Actor portrayal
Actor portrayal
*KONFIDENT-S is a multicenter, open-label extension trial of 134 adult and adolescent (≥12 years of age) patients. Study participants had a confirmed diagnosis of HAE and ≥2 attacks within 3 months and were enrolled after completing the KONFIDENT phase 3 trial or de novo.8,9
†For severe laryngeal attacks, patients were directed to treat with their conventional on-demand medication.10
HAE=hereditary angioedema.
References:
1. Valerieva A, Longhurst HJ. Treatment of hereditary angioedema—single or multiple pathways to the rescue.
Front Allergy. 2022;3:952233. doi:10.3389/falgy.2022.952233 2. Riedl MA, Farkas H, Aygören-Pürsün E, et al.
Oral sebetralstat for on-demand treatment of hereditary angioedema attacks. N Engl J Med.
2024;391(1):32-43. doi:10.1056/NEJMoa2314192 3. EKTERLY. Package insert. KalVista Pharmaceuticals,
Inc.; 2025. 4. Riedl MA, Farkas H, Aygören-Pürsün E, et al. Oral sebetralstat for on-demand
treatment of hereditary angioedema attacks. N Engl J Med. 2024;391(1)(suppl 1):1-33.
doi:10.1056/NEJMoa2314192 5. Riedl MA, Farkas H, Aygören-Pürsün E, et al. Oral sebetralstat for
on-demand treatment of hereditary angioedema attacks. N Engl J Med. 2024;391(1)(protocol):1-263.
doi:10.1056/NEJMoa2314192 6. Cohn DM, Aygören-Pürsün E, Bernstein JA, et al. Evaluation of
patient-reported outcome measures for on-demand treatment of hereditary angioedema attacks and
design of KONFIDENT, a phase 3 trial of sebetralstat. Clin Transl Allergy. 2023;13(9):e12288.
doi:10.1002/clt2.12288 7. Cohn DM, Soteres DF, Craig TJ, et al. Interplay between on-demand
treatment trials for hereditary angioedema and treatment guidelines. J Allergy Clin Immunol.
2025;155(3):726-739. doi:10.1016/j.jaci.2024.12.1079 8. Data on File. KalVista Pharmaceuticals, Inc.
2024. 9. Data on File. KalVista Pharmaceuticals, Inc. 2024. 10. Data on File. KalVista
Pharmaceuticals, Inc. 2024.
EKTERLY® (sebetralstat) is a plasma kallikrein inhibitor indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adult and pediatric patients aged 12 years and older.
Adverse reactions: The most commonly reported adverse reaction was headache.
Drug interactions: EKTERLY is a substrate of CYP3A4. Concomitant use of EKTERLY with a strong CYP3A4 inhibitor increases sebetralstat exposure, which may increase the risk of sebetralstat adverse reactions. Avoid use of EKTERLY with strong CYP3A4 inhibitors and reduce the dose of EKTERLY to one dose of 300 mg (one tablet) with moderate CYP3A4 inhibitors. Concomitant use of EKTERLY with a strong or moderate CYP3A4 inducer decreases sebetralstat exposure, which may decrease efficacy. The use of EKTERLY with strong or moderate CYP3A4 inducers is not recommended.
Use in specific populations: Avoid use of EKTERLY in patients with severe hepatic impairment (Child-Pugh Class C). The recommended dosage of EKTERLY is one dose of 300 mg (one tablet) in patients with moderate hepatic impairment (Child-Pugh Class B).
There are no available data on EKTERLY in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. There are no data on the presence of sebetralstat or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production.
The safety and effectiveness of EKTERLY in pediatric patients aged under 12 years of age have not been established.
To report SUSPECTED ADVERSE REACTIONS, contact KalVista Pharmaceuticals, Inc. at 1-855-258-4782 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.