You are about to leave EKTERLYHCP.com
You will be redirected to a third-party website
not affiliated with KalVista Pharmaceuticals.
This website is intended for US healthcare professionals.
No matter where a hereditary
angioedema (HAE) attack occurs,

The first and only oral on-demand treatment that
helps your patients stop HAE attacks fast2-4
The ability to treat
The first and only oral HAE treatment that allows for treatment at attack recognition2,4
Explore dosing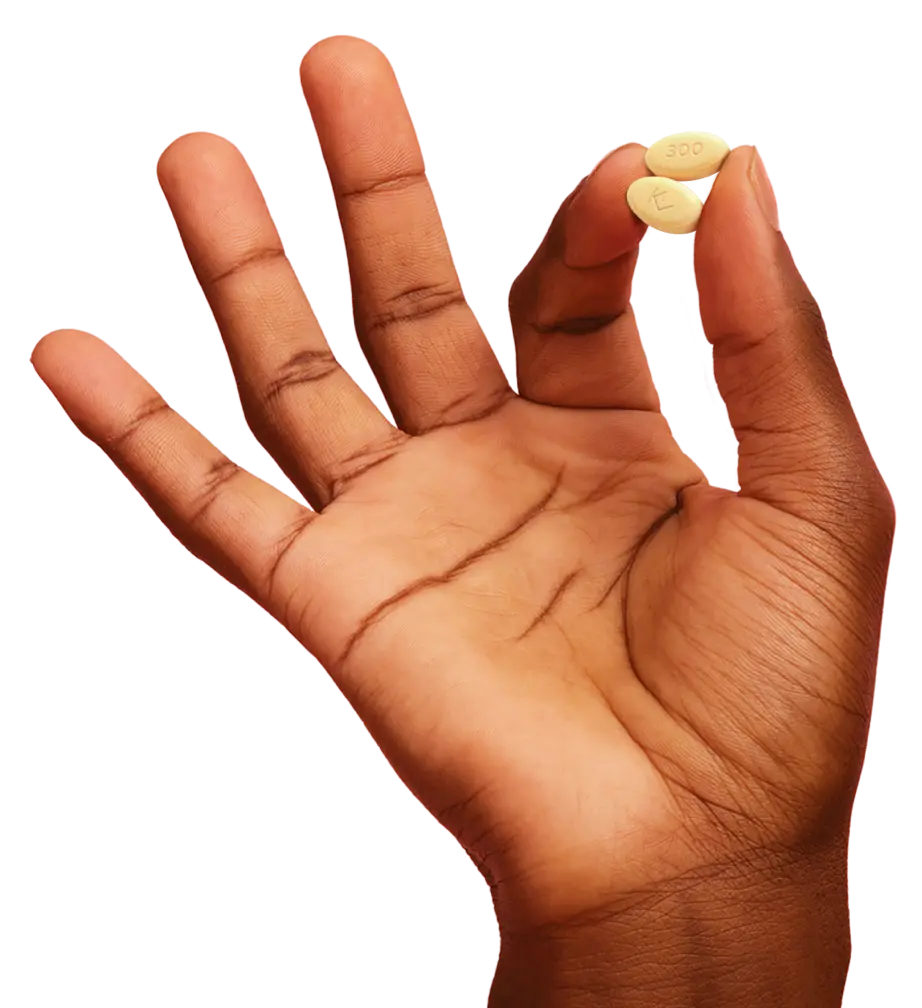
Learn about personalized support your
patients can rely on.

Connect with a KalVista representative to stay up to date with EKTERLY.
References:
1. Riedl MA, Farkas H, Aygören-Pürsün E, et al. Oral sebetralstat for on-demand treatment of hereditary angioedema attacks. N Engl J Med. 2024;391(1):32-43. doi:10.1056/NEJMoa2314192
2. EKTERLY. Package insert. KalVista Pharmaceuticals, Inc.; 2025.
3. Data on File. KalVista Pharmaceuticals, Inc. 2024.
4. KalVista Pharmaceuticals announces FDA approval of EKTERLY (sebetralstat), first and only oral on demand treatment for hereditary angioedema. Published July 2025. Accessed July 2025.
EKTERLY® (sebetralstat) is a plasma kallikrein inhibitor indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adult and pediatric patients aged 12 years and older.
Adverse reactions: The most commonly reported adverse reaction was headache.
Drug interactions: EKTERLY is a substrate of CYP3A4. Concomitant use of EKTERLY with a strong CYP3A4 inhibitor increases sebetralstat exposure, which may increase the risk of sebetralstat adverse reactions. Avoid use of EKTERLY with strong CYP3A4 inhibitors and reduce the dose of EKTERLY to one dose of 300 mg (one tablet) with moderate CYP3A4 inhibitors. Concomitant use of EKTERLY with a strong or moderate CYP3A4 inducer decreases sebetralstat exposure, which may decrease efficacy. The use of EKTERLY with strong or moderate CYP3A4 inducers is not recommended.
Use in specific populations: Avoid use of EKTERLY in patients with severe hepatic impairment (Child-Pugh Class C). The recommended dosage of EKTERLY is one dose of 300 mg (one tablet) in patients with moderate hepatic impairment (Child-Pugh Class B).
There are no available data on EKTERLY in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. There are no data on the presence of sebetralstat or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production.
The safety and effectiveness of EKTERLY in pediatric patients aged under 12 years of age have not been established.
To report SUSPECTED ADVERSE REACTIONS, contact KalVista Pharmaceuticals, Inc. at 1-855-258-4782 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.